Calculate the Composition in Weight Percent of an Alloy
A What is the composition in weight percent of an alloy that consists of 6 at Pb and 94 at Sn. Of y in the same manner by recalculating after substituting all the xs and ys or by simply subtracting the Wt.

Solved Calculate The Composition In Weight Percent Of An Chegg Com
Find step-by-step Engineering solutions and your answer to the following textbook question.

. Calculate the composition in weight percent of an alloy that contains 2180 kg titanium 146 kg of aluminum and 97 kg of vanadium. An equimolar mixture of H2 and D2 effuses through an. The Atomic weights of.
Calculate the weight percent of an alloy component given a desired average density. Calculate the composition in weight percent of an alloy that contains 2180 kg titanium 146 kg of aluminum and 97 kg of vanadium. Saying that 1mL of each 2mL total mass of Al 271 27 grams mass of Li 05341 0534 grams mass of the entire system 2552 51 grams putting that into the.
This is pretty easy all we have to do is to sum the mass of each element in the alloy and then divide each mass between the total to get the percentage in weight. Calculate the composition in weight percent of an alloy that contains 2180 kg titanium 146 kg of aluminum and 97 kg of vanadium. In a metallic alloy system the compositions of 2 or more metals may be expressed as weight percentage or as atomic percentage.
For the composition of irony isnt going to 98 point 87. Chemistry questions and answers. It is easy to calculate the At.
However the weight percentage is more usual system. Apb 2072 amu. Mechanical Engineering questions and answers.
Answer The concentration in weight percent of an. Of x as determined above from. For each master alloy eg.
Mass mass of element in 1 mole of the compound molar mass of the compound x 100 or mass percent mass of solute mass of solution x 100 The units of. For example there is a supplier who sells commercial master alloys and on their website they have various compositions of such. Let us assume xNoof Ni atoms with yAtoms of P.
What is the composition in weight percent of an alloy that consists of 6 at Pb and 94 at Sn. If the mass fraction of the liquid phase is 068. Mechanical Engineering questions and answers.
Calculate an average density of alloy. The carbon divide by Must have called. A coppersilver alloy is heated to 90 0 C 900circ mathrmC 90 0 C and is found to consist of α alpha α and liquid phases.
Solution The concentration in weight percent of an. Then Nommol NiNommol P xy. You have to know the Stoichiometric relation between Ni and P in the Compound making the Alloy.
A Calculate the composition in weight percent of an alloy that contains 2180 kg titanium 146 kg of aluminum and 97 kg of vanadium. 416 Calculate the composition in weight percent of an alloy that contains 105 kg of iron 02 kg of carbon and 10 kg of chromium. B What is the composition in weight percent of an alloy that consists of 218 kg of.
Calculate the composition in weight percent of an alloy that contains 90 kg of Fe 20 kg of Cr and 15 kg of Ni. Calculate the composition in weight percent of an alloy that contains 105 kg of Fe 02 kg of. Calculate the composition in weight percent of an alloy that contains 2180 mathrmkg titanium 146 kg aluminum and 97 mathrmkg vanadium.
Q2 Calculate the composition in weight percent and in atom percent of an alloy that contains 315 kg Titanium 545 kg Aluminum and 563 kg Vanadium. Iron M 5584 gmol σ_a256 b σ_s112 b carbon M 12. ρ764 gcm3 Σ_a205 cm-1 Σ_s956cm-1 It has properties of two elements.
And similarly similarly for carbon we can write the composition of carbon as it went to myself. There is an alloy.

Solved Calculate The Composition In Weight Percent Of An Chegg Com

Solved Question 1 Calculate The Composition In Weight Chegg Com

Solved G Calculate The Composition In Weight Percent Of Chegg Com
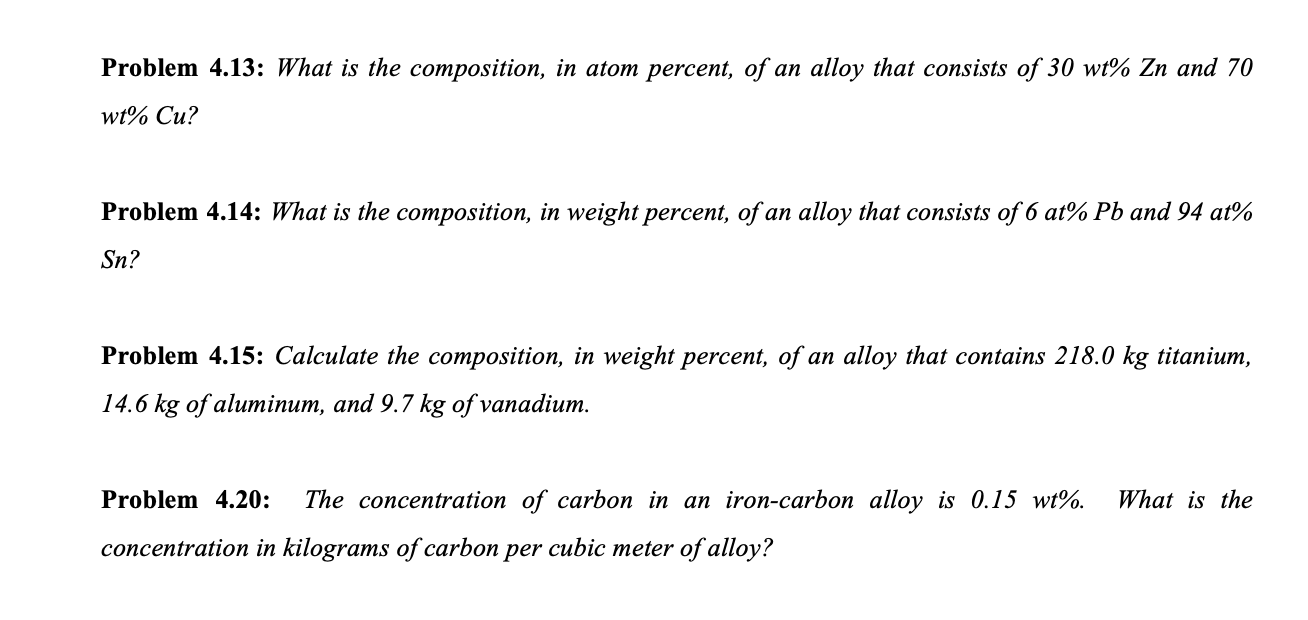
Solved Problem 4 13 What Is The Composition In Atom Chegg Com
No comments for "Calculate the Composition in Weight Percent of an Alloy"
Post a Comment